Posts Tagged: sodium
Sodium Adsorption Ratio (SAR), Briefly
I look at a lot of soil reports these days, and the sodium adsorption ratio (SAR) is one number that is very informative to me.
Simply put, SAR of a soil extract takes into account how much the adverse effect of sodium (Na+) is moderated by the other cations calcium (Ca2+) and magnesium (Mg2+). As you all know, calcium and magnesium can replace sodium on soil particles, subsequently permitting this toxin to be washed away from the plant roots. So the more free calcium and magnesium we have around in the soil, the better odds we have of mitigating sodium.
SAR levels below 6 are OK, levels which run above 10 mean trouble.
Although most soil reports will give you a calculated value for SAR, you can calculate SAR yourself. It's simple: SAR = Na+/((1/2(Ca2+ + Mg2+))1/2 ), where Na+, Ca2+ and Mg2+ are all measured in meq/L (milliequivalents per liter).
Understanding the Sodium Results on a Soil Analysis
I am writing this in response to the questions at yesterday's mineral nutrition meeting regarding the two different outputs on laboratory soil analyses for sodium. Pretty tough gig standing in front of about 70 of your peers and not having the answer right away, but so it goes.
The questions pertained to the difference between a reading of “exchangeable sodium” measured in parts per million (ppm) or mg/kg, and sodium measured in meq/l (milliequivalents per litre) from saturated paste. The photos below of three different soil samples tested by a local analysis provider shows the “exchangeable sodium” output in mg/kg (also read as ppm) on the right, and sodium measured in meq/L from saturated paste on the left.
Let's summarize a recent communication of mine with Dr. Tim Hartz from UC Davis in order to understand what these mean to us:
1. The extract from NH4OAc method is the "exchangeable sodium" a minority of which is considered to be ‘active' -meaning what the plant root could encounter- in the soil solution. The "exchangeable sodium percentage" (ESP) for coastal soils is around 2.5% of the base saturation on average, and one shouldn't run into trouble until this percentage of exchangeable sodium gets in the range of 7 or 8%.
2. To see what is ‘active' and what the plant root is facing, one needs to look at the saturated paste analysis which measures soluble sodium. Numbers around 3 or 4 meq/liter are good and don't indicate a problem; numbers much higher than these figures would be point to a sodium problem.
Let's go to the analyses below from some soils and practice with these numbers for exchangeable sodium and saturated paste extracts measuring soluble sodium.
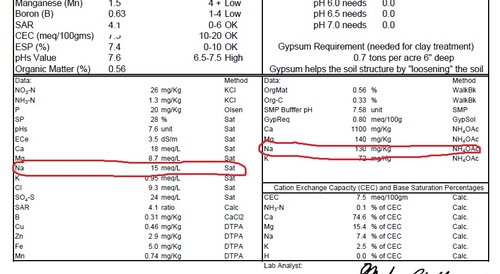
Sample # 1: Saturation extract of Na measured as 15 meq/L, and the amount of 'exchangeable sodium' of 130 mg/kg (ppm) is 7.4% of the total base saturation (box at bottom right) , well above the 2.5% which is OK for coastal soils . The strawberry plants from this area of the field were dead.
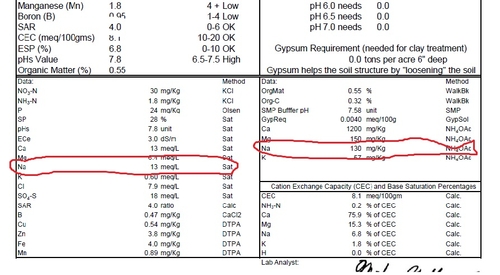
Sample #2: Saturation extract of Na measured as 13 meq/L, and the amount of 'exchangeable sodium' of 130 mg/kg is 6.8% of the total base saturation (box at bottom right) , well above the 2.5% which is OK for coastal soils. The strawberry plants from this part of the field were dying.
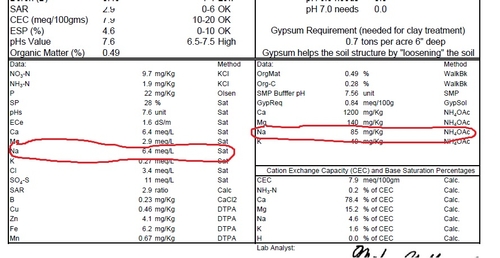
Sample #3: Saturation extract of Na measured as 6.4 meq/L, and the amount of 'exchangeable sodium' of 85 mg/kg (ppm) is 4.6% (box at bottom right) of the base saturation; seems OK. The plants from this area of the field were apparently all right.
Approaches to Preventing Strawberry Yellowing in Sodium and Chloride Affected Soils
The following is an addition to the post from August 2 concerning a case of yellowing strawberry plants in a field in Castroville. To summarize, we concluded that the plants are turning yellow because they are being poisoned by excess amounts of sodium and chloride accumulating in the bed.
So of course, and indeed the question was asked right away, what can we do about this problem? The three points below are a start:
1. The dramatic accumulation of sodium and chloride at the surface of the bed in this study indicate that these are not being adequately leached, which was verified by our observation of soil saturation at depths below the bed. If this is correct, by increasing drainage we will be able to ameliorate the situation and restore a normal leaching pattern. Short of installing a system of subsurface drainage, there has been some anecdotal evidence of success in using a certain “Yeoman’s plough” which is essentially a shank going some 16 inches deep into the soil next to the bed opening a deep cut in the soil improving aeration and water infiltration. This has not been tested in replicated trials however, so I can’t make a firm recommendation of this method at this time.
2. We have every indication in this study that substantial amounts of calcium are precipitating out as lime, and is therefore not replacing exchangeable sodium. We could reduce the amount of precipitation of calcium (increasing the exchangeable calcium) by acidifying the irrigation water which would go some lengths to mitigate the sodium hazard (SAR).
3. Finally, there are other useful steps which can be taken to reduce the amount of sodium and chloride being introduced into the field. For example, a grower experiencing a situation of high sodium and chloride should avoid using sodium nitrate or potassium chloride fertilizers.

Field of yellowed strawberry plants in a chloride and sodium affected field in Castroville.
Arrival at a Probable Cause of Yellow Strawberry Plants in Castroville
Introduction: The following is a description of a second, more thorough evaluation of the situation described previously in this space of yellow strawberry plants in a field in Castroville. This issue of yellow strawberry plants popping up in certain locations in the Castroville – Salinas production area has confounded us for years, but I believe the work described below allows us to make a strong argument as to the cause is of this malady at least in this case.
Materials and Methods: A total of four samples (two from an area of severe yellowing, and two from an area of apparently healthy green plants) were taken. Following the output example posted below (Figure 2), each sample consists of 10 zones of a bed, and each zone is tested for 14 parameters. At each soil sampling site, a representative plant sample was uprooted and taken away for analysis of tissue mineral concentration.
Results: The data in the Tables 1 and 2 below represent an average of the two samples taken each for yellow and green plants. In order to better interpret the data, several zones have been grouped together. Zones 9 and 10 represent the surface of the bed, zones 1, 2 and 3 represent the soil straight underneath the drip tape, zone 8 the plant zone, zone 7 the root zone and zones 6, 5 and 4 being underneath the root zone 7.
Table 1: Evaluation of Zones 1- 6 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zones 1,2 and 3 |
Zones 4,5 and 6 |
||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
33.7 |
33.6 |
29.8 |
31.8 |
|
pH (units) |
8.5 |
8.1 |
8.5 |
8.1 |
|
EC5 (umhos/cm) |
269 |
267 |
381 |
355 |
|
Ammonia (NH3-N) |
24 |
32 |
60 |
16 |
|
Nitrate (NO3) |
204 |
49 |
443 |
94 |
|
Phosphate (PO4) |
126 |
86 |
66 |
68 |
|
Potassium (K) |
157 |
184 |
157 |
92 |
|
Calcium (Ca) |
419 |
417 |
398 |
201 |
|
Magnesium (Mg) |
169 |
375 |
174 |
130 |
|
Carbonate (CaCO3) % |
2.3 |
1.8 |
2.7 |
1.7 |
|
Sulfate (SO4) |
219 |
195 |
694 |
412 |
|
Sodium (Na) |
454 |
417 |
578 |
426 |
|
Chloride (Cl) |
175 |
184 |
181 |
242 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
Table 2: Evaluation of Zones 7-10 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zone 7- Root Zone |
Zone 8- Plant Zone |
Zones 9 and 10 |
|||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
30.6 |
32.1 |
33 |
33.1 |
22.8 |
26.3 |
|
pH (units) |
8.7 |
8.2 |
8.7 |
8.3 |
8.3 |
8.2 |
|
EC5 (umhos/cm) |
305 |
389 |
258 |
374 |
938 |
546 |
|
Ammonia (NH3-N) |
34 |
14 |
54 |
16 |
48 |
44 |
|
Nitrate (NO3) |
314 |
30 |
199 |
25 |
968 |
452 |
|
Phosphate (PO4) |
6.5 |
57 |
85 |
55 |
111 |
45 |
|
Potassium (K) |
144 |
74 |
185 |
89 |
206 |
89 |
|
Calcium (Ca) |
341 |
156 |
713 |
194 |
512 |
195 |
|
Magnesium (Mg) |
140 |
83 |
383 |
127 |
384 |
104 |
|
Carbonate (CaCO3) % |
2 |
1.3 |
2.0 |
1.9 |
2.0 |
1.9 |
|
Sulfate (SO4) |
434 |
492 |
239 |
437 |
1216 |
622 |
|
Sodium (Na) |
500 |
446 |
492 |
496 |
872 |
520 |
|
Chloride (Cl) |
160 |
309 |
165 |
330 |
433 |
310 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
0 |
0 |
Table 3: Comparison of Mineral Concentrations of Leaf Tissue for Green and Yellow Plants
|
Mineral |
Yellow Plant |
Green Plant |
|
Total Nitrogen |
2.4% |
2.2% |
|
Total Phosphorous |
0.38% |
0.44% |
|
Potassium |
1.1% |
1.2% |
|
Calcium |
1.5% |
1.3% |
|
Magnesium |
0.55% |
0.38% |
|
Total Sulfur |
0.21% |
0.18% |
|
Copper |
4.5 ppm |
3.7 ppm |
|
Zinc |
23 ppm |
18 ppm |
|
Iron |
515 ppm |
365 ppm |
|
Manganese |
185 ppm |
108 ppm |
|
Boron |
73 ppm |
78 ppm |
|
Molybdenum |
1.1 ppm |
1.9 ppm |
|
Sodium |
350 ppm |
79 ppm |
|
Chloride |
4150 ppm |
3000 ppm |
I’ve also taken a look at the irrigation water. As is common in northern Monterey county, the farm gets its water as a mixture of recycled water blended with well or river water. A full report for an example of the blended irrigation water used on this farm is available from the Monterey Regional Water Pollution Control Agency at:
http://www.mrwpca.org/recycling/chem2012_blended.php
In the sample taken of the blended recycled and river water mix, conductivity (EC) was 1.3 dS/m, sodium 118 ppm, chloride 160 ppm and adjusted SAR of 3.4 (sodium adsorption ratio, an index of sodium hazard adjusted for the amount of calcium in the irrigation water).
Discussion: The pH of the soil in the beds in all zones is quite high, which is not surprising because of the high percentage of carbonates (lime) throughout. One can also see accumulations of nitrates, phosphates and potassium are substantially higher in the areas of the yellow plants, which quite likely due to their declining ability to take up the nutrients being continually applied as fertilizer. It is worth noting that nitrates in the high concentrations found in our soil tests can be toxic to plants, thus accelerating the decline of plants.
Nitrites, generated from ammonium in anaerobic conditions, are zero, indicating adequate aeration in the bed.
In terms of the irrigation water, referring to the water quality guidelines for crops developed by UC Cooperative Extension, we find that the water used on this farm can be used with some restriction to irrigate crops moderately susceptible to salinity such as strawberry. In other words, this irrigation water isn’t great but is OK.
It seems that the real culprits in this field are the accumulated amounts of chloride and sodium. Generally speaking, crops in our area perform best when the soil sodium levels are less than 250 ppm and soil chloride levels are less than 100 ppm.
Across all the soil samples the average amounts of sodium and chloride are above 250 ppm and 100 ppm respectively. High as they are, the concentrations of both ions do not vary greatly in the plant and root zones and the zones around them in either areas of green or yellow plants. There are, however, substantial differences in the concentrations of these ions at the crust (zones 9 and 10) between green and yellow plant areas. For example, on average soil from areas of yellow plants there is nearly a twofold accumulation of sodium in the crust of soil taken as well as a substantially higher amount of chloride over the soil sampled around green, healthy plants. This tells us that while these large amounts of sodium and chloride (also known as salts) are accumulating away from the plant via evaporation, they are nevertheless passing through more sensitive plant zones to get there. It is not difficult then to hypothesize that during this transition the yellow plants are picking up these salts obtaining the fourfold accumulation of sodium and 40% increase in chlorides that we observe in the leaf tissue of yellow plants over that of green.
Furthermore, it is notable that the amount of lime (CaCO3) in all the soil samples is high. This indicates that a lot of calcium coming into the soil from irrigation water is precipitating out and not as capable of sufficiently limiting the amount of exchangeable sodium. In turn the sodium hazard is high. This may also explain to some extent why areas quite close to one another respond differently, since in some areas (perhaps even quite close together) more calcium precipitates out than others.
My conclusion drawn from all this work in this particular instance of severe plant yellowing of strawberry plants is that the uptake of sodium and chloride by these affected plants is very high and they are being poisoned by salt.
Thank you to Frank Shields and Soil Control Lab for their generous assistance in this work. Thanks to other colleagues for their valuable insight and input which assisted me in the development of my conclusion.
This project was supported in part with funds provided by the California Strawberry Commission.

Picture of yellow strawberry plants in Castroville field. Note washed out, almost bleached appearance coupled with uneven distribution in affected area.
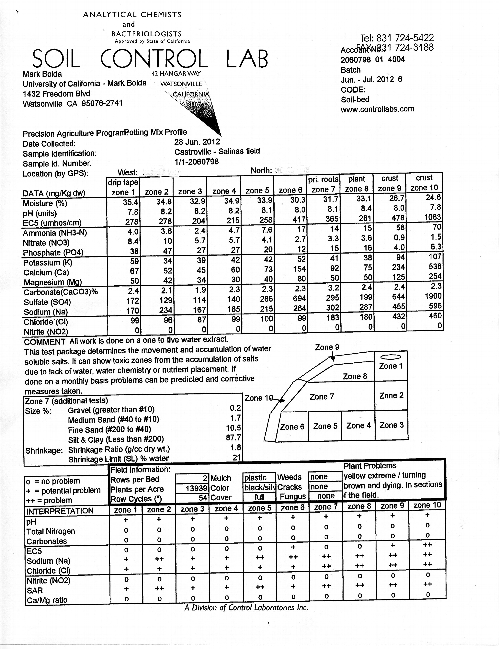
Example of output from soil tests from ten distinct zones of the bed, use graphic to understand Tables 1-2 above.
Closing in on an Answer on a Case of Yellow Strawberry Plants in Castroville.
Situation: The complaint we were invited to evaluate in this field was less typical than what one often finds with yellow plants in Salinas or Watsonville. These patches of yellow plants were typically dispersed in patches of various sizes in the field (photo 1 below), but on one side of the field this was particularly pronounced. This area corresponded with a farm road from a previous artichoke plantation as well as being the end of the drip tapes installed by the strawberry grower.
The bed tops tended to be dry and moisture has been adequate but not excessive, so my running thesis of excess water actually did not fit so well in this situation.
I had the good fortune on this particular call of being accompanied by Frank Shields from Soil Control Lab, who did a thorough evaluation of a bed and plant tissue (photo 2 below) and arrived at the results as depicted in photos 3 and 4 below.
Evaluation: As readers can assess from the attached analyses, this sampling was extraordinarily detailed. The soil analysis breaks down the bed into ten zones and evaluates no less than 14 parameters of each zone. Furthermore, in zone 7, Frank took a general sample of the soil texture and shrinkage, which gives an assessment of at what moisture percentage cracks form in the soil.
As a standard operating procedure, Frank sampled one bed containing symptomatic plants according to a pattern of ten different areas, the cross section of which can be seen in the soil report attached below. Additionally, Frank uprooted several plants for evaluation of the roots and tissue mineral contents in the foliage.
This soil test strives to accomplish a number of things. First, it is designed to monitor the movement and location of accumulated soluble salts for the purpose of determining the water pattern from the irrigation system. Similarly, since during the season strawberry fertilizer is supplemented via the water it is useful to know where the nutrients are ending in the bed to confirm that they are getting to the plant and not being leached out of range or being affected by adverse pH or salt conditions.
Plant available nutrients are both water soluble and exchangeable. This analysis is designed to monitor the water soluble fraction for the water soluble plant nutrients nitrate, nitrite, chloride, and sulfate as well as cations which accumulate with the water soluble fraction like calcium, potassium, sodium and ammonia.
Interpretation of Results:
Moisture: All zones were very wet indicating recent irrigation of sufficient volume to completely fill the bed and should leach salts and nutrients down and out of the bed. Additionally, there were no shrinkage cracks visible when observing the fifty foot section of row again indicating that the soil had been kept sufficiently moist.
The pH is on the high side and evenly distributed between 7.8 and 8.4 though all zones. However, this pH is not unusually high and normally would not be said to be problematic for strawberries.
Soil potassium is this sample low. It is typical for soils in Salinas and the Pajaro Valley to have potassium concentrations above 150 ppm, but with the exception of zone 10 of the bed this particular sample ranges around 50 ppm or below. This is reflected in the leaf potassium concentration of 0.9% when a range of 1.3 – 1.8% is ideal for this time of year.
Soil nitrate appears to be substantially leached out, and indeed the percentage in the leaves of the sampled yellow plants is 2.0%, which is somewhat less than the optimum of 2.4- 3.0 % for this time of year. Still, 2.0 % dry weight tissue nitrogen would not explain the substantial yellowing we observe in the field.
Phosphorous, calcium, magnesium and the other micronutrients appear to be sufficient according to the attached tissue analysis.
Nitrites, which are oxidized to plant available nitrate in the process of nitrification but toxic to plants in quantity are absent from the sample. This is quite probably because of the well aerated soil and lack of packing.
EC5: This is a measure of the amount of water soluble components in a zone. Zone six has the highest in the root zones (1 – 8) but all within values to support plant growth. With proper watering, salts should accumulate in zone six, nine and ten. In this sample, salt accumulation is highest at zone ten typical of a pattern of a bed having full mulch and plenty of water.
We can also look at the ratio of water soluble cations to get an idea on the ratio of plant available constituents like the SAR value and soluble Ca/Mg ratio. The very high sodium and chloride compared to the low calcium and magnesium in this sample is significant and indicates a problem. Indeed, sodium and chloride are excessively high in the plant tissue and very likely to be interfering with normal plant function.
Outside of the carbonates, the soil minerals are an accumulation both from the irrigation water and nutrients added to the soil. If most of the salts accumulated in the bed are naturally found in the irrigation water (as is the case here), it means that the nutrients are being leached out of the root zone with excess water and additional fertility could be considered.
Conclusion: Rather than being a problem of the deficiencies of nitrogen or potassium, Frank maintains that at issue here is a buildup in the affected areas of chloride and accumulated salts as a result of irrigation. Carrying this thesis further, perhaps if the amount of current irrigation water could be limited in the areas experiencing yellow plants, one would also be reducing the amounts of apparently damaging sodium and chloride. That might be one way to address the problem.
The scattershot pattern of yellow plants across the field is the major confounding point of the problem being evaluated here. On the one hand it would indicate that the toxicities and deficiencies described above are occurring in the same scattershot pattern across the field but it does beg the question why the differences are so dramatic, sometimes even from one plant to the next.
So, while this evaluation has given us a good look at what is going on around these yellow plants, we still don't conclusively know what exactly is the cause of this problem. It absolutely merits further work.
Thank you to the grower who invited us out. I thank Frank Shields of Soil Control Lab for the contribution of his time and expertise to working on this problem.

View of the situation. Yellow plants scattered among healthy green plants. Note healthy green plant in the middle of a lot of yellow ones in the bottom center of the photograph.

Frank Shields from Soil Control Lab sampling soil and plants.

Tissue Analysis of Yellow Plant Courtesy of Soil Control Lab
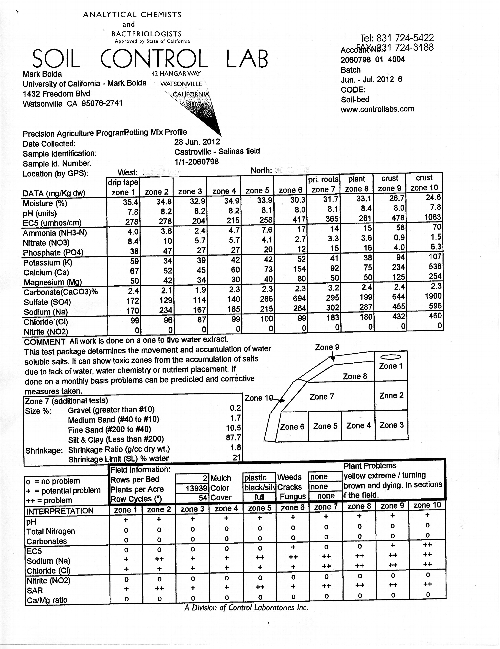
Soil Evaluation Courtesy Soil Control Lab. The diagram to the center right is a cross section of half a bed, divided into ten parts.

