Arrival at a Probable Cause of Yellow Strawberry Plants in Castroville
Introduction: The following is a description of a second, more thorough evaluation of the situation described previously in this space of yellow strawberry plants in a field in Castroville. This issue of yellow strawberry plants popping up in certain locations in the Castroville – Salinas production area has confounded us for years, but I believe the work described below allows us to make a strong argument as to the cause is of this malady at least in this case.
Materials and Methods: A total of four samples (two from an area of severe yellowing, and two from an area of apparently healthy green plants) were taken. Following the output example posted below (Figure 2), each sample consists of 10 zones of a bed, and each zone is tested for 14 parameters. At each soil sampling site, a representative plant sample was uprooted and taken away for analysis of tissue mineral concentration.
Results: The data in the Tables 1 and 2 below represent an average of the two samples taken each for yellow and green plants. In order to better interpret the data, several zones have been grouped together. Zones 9 and 10 represent the surface of the bed, zones 1, 2 and 3 represent the soil straight underneath the drip tape, zone 8 the plant zone, zone 7 the root zone and zones 6, 5 and 4 being underneath the root zone 7.
Table 1: Evaluation of Zones 1- 6 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zones 1,2 and 3 |
Zones 4,5 and 6 |
||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
33.7 |
33.6 |
29.8 |
31.8 |
|
pH (units) |
8.5 |
8.1 |
8.5 |
8.1 |
|
EC5 (umhos/cm) |
269 |
267 |
381 |
355 |
|
Ammonia (NH3-N) |
24 |
32 |
60 |
16 |
|
Nitrate (NO3) |
204 |
49 |
443 |
94 |
|
Phosphate (PO4) |
126 |
86 |
66 |
68 |
|
Potassium (K) |
157 |
184 |
157 |
92 |
|
Calcium (Ca) |
419 |
417 |
398 |
201 |
|
Magnesium (Mg) |
169 |
375 |
174 |
130 |
|
Carbonate (CaCO3) % |
2.3 |
1.8 |
2.7 |
1.7 |
|
Sulfate (SO4) |
219 |
195 |
694 |
412 |
|
Sodium (Na) |
454 |
417 |
578 |
426 |
|
Chloride (Cl) |
175 |
184 |
181 |
242 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
Table 2: Evaluation of Zones 7-10 of Bed in Yellow and Healthy Areas
|
Data (mg/Kg dw) |
Zone 7- Root Zone |
Zone 8- Plant Zone |
Zones 9 and 10 |
|||
|
|
Yellow |
Healthy |
Yellow |
Healthy |
Yellow |
Healthy |
|
Moisture (%) |
30.6 |
32.1 |
33 |
33.1 |
22.8 |
26.3 |
|
pH (units) |
8.7 |
8.2 |
8.7 |
8.3 |
8.3 |
8.2 |
|
EC5 (umhos/cm) |
305 |
389 |
258 |
374 |
938 |
546 |
|
Ammonia (NH3-N) |
34 |
14 |
54 |
16 |
48 |
44 |
|
Nitrate (NO3) |
314 |
30 |
199 |
25 |
968 |
452 |
|
Phosphate (PO4) |
6.5 |
57 |
85 |
55 |
111 |
45 |
|
Potassium (K) |
144 |
74 |
185 |
89 |
206 |
89 |
|
Calcium (Ca) |
341 |
156 |
713 |
194 |
512 |
195 |
|
Magnesium (Mg) |
140 |
83 |
383 |
127 |
384 |
104 |
|
Carbonate (CaCO3) % |
2 |
1.3 |
2.0 |
1.9 |
2.0 |
1.9 |
|
Sulfate (SO4) |
434 |
492 |
239 |
437 |
1216 |
622 |
|
Sodium (Na) |
500 |
446 |
492 |
496 |
872 |
520 |
|
Chloride (Cl) |
160 |
309 |
165 |
330 |
433 |
310 |
|
Nitrite (NO2) |
0 |
0 |
0 |
0 |
0 |
0 |
Table 3: Comparison of Mineral Concentrations of Leaf Tissue for Green and Yellow Plants
|
Mineral |
Yellow Plant |
Green Plant |
|
Total Nitrogen |
2.4% |
2.2% |
|
Total Phosphorous |
0.38% |
0.44% |
|
Potassium |
1.1% |
1.2% |
|
Calcium |
1.5% |
1.3% |
|
Magnesium |
0.55% |
0.38% |
|
Total Sulfur |
0.21% |
0.18% |
|
Copper |
4.5 ppm |
3.7 ppm |
|
Zinc |
23 ppm |
18 ppm |
|
Iron |
515 ppm |
365 ppm |
|
Manganese |
185 ppm |
108 ppm |
|
Boron |
73 ppm |
78 ppm |
|
Molybdenum |
1.1 ppm |
1.9 ppm |
|
Sodium |
350 ppm |
79 ppm |
|
Chloride |
4150 ppm |
3000 ppm |
I’ve also taken a look at the irrigation water. As is common in northern Monterey county, the farm gets its water as a mixture of recycled water blended with well or river water. A full report for an example of the blended irrigation water used on this farm is available from the Monterey Regional Water Pollution Control Agency at:
http://www.mrwpca.org/recycling/chem2012_blended.php
In the sample taken of the blended recycled and river water mix, conductivity (EC) was 1.3 dS/m, sodium 118 ppm, chloride 160 ppm and adjusted SAR of 3.4 (sodium adsorption ratio, an index of sodium hazard adjusted for the amount of calcium in the irrigation water).
Discussion: The pH of the soil in the beds in all zones is quite high, which is not surprising because of the high percentage of carbonates (lime) throughout. One can also see accumulations of nitrates, phosphates and potassium are substantially higher in the areas of the yellow plants, which quite likely due to their declining ability to take up the nutrients being continually applied as fertilizer. It is worth noting that nitrates in the high concentrations found in our soil tests can be toxic to plants, thus accelerating the decline of plants.
Nitrites, generated from ammonium in anaerobic conditions, are zero, indicating adequate aeration in the bed.
In terms of the irrigation water, referring to the water quality guidelines for crops developed by UC Cooperative Extension, we find that the water used on this farm can be used with some restriction to irrigate crops moderately susceptible to salinity such as strawberry. In other words, this irrigation water isn’t great but is OK.
It seems that the real culprits in this field are the accumulated amounts of chloride and sodium. Generally speaking, crops in our area perform best when the soil sodium levels are less than 250 ppm and soil chloride levels are less than 100 ppm.
Across all the soil samples the average amounts of sodium and chloride are above 250 ppm and 100 ppm respectively. High as they are, the concentrations of both ions do not vary greatly in the plant and root zones and the zones around them in either areas of green or yellow plants. There are, however, substantial differences in the concentrations of these ions at the crust (zones 9 and 10) between green and yellow plant areas. For example, on average soil from areas of yellow plants there is nearly a twofold accumulation of sodium in the crust of soil taken as well as a substantially higher amount of chloride over the soil sampled around green, healthy plants. This tells us that while these large amounts of sodium and chloride (also known as salts) are accumulating away from the plant via evaporation, they are nevertheless passing through more sensitive plant zones to get there. It is not difficult then to hypothesize that during this transition the yellow plants are picking up these salts obtaining the fourfold accumulation of sodium and 40% increase in chlorides that we observe in the leaf tissue of yellow plants over that of green.
Furthermore, it is notable that the amount of lime (CaCO3) in all the soil samples is high. This indicates that a lot of calcium coming into the soil from irrigation water is precipitating out and not as capable of sufficiently limiting the amount of exchangeable sodium. In turn the sodium hazard is high. This may also explain to some extent why areas quite close to one another respond differently, since in some areas (perhaps even quite close together) more calcium precipitates out than others.
My conclusion drawn from all this work in this particular instance of severe plant yellowing of strawberry plants is that the uptake of sodium and chloride by these affected plants is very high and they are being poisoned by salt.
Thank you to Frank Shields and Soil Control Lab for their generous assistance in this work. Thanks to other colleagues for their valuable insight and input which assisted me in the development of my conclusion.
This project was supported in part with funds provided by the California Strawberry Commission.

Picture of yellow strawberry plants in Castroville field. Note washed out, almost bleached appearance coupled with uneven distribution in affected area.
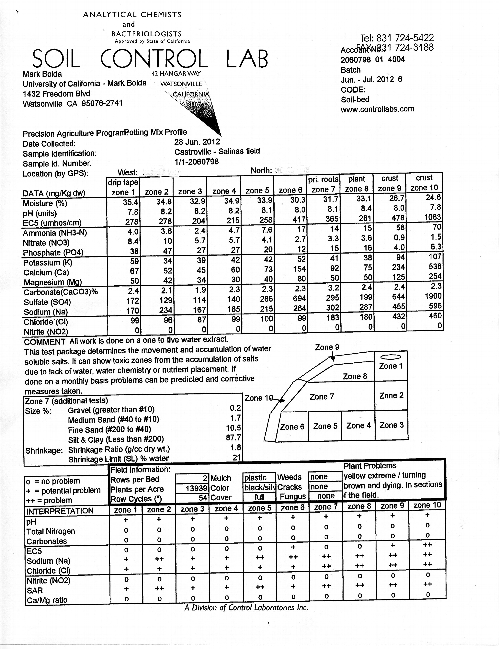
Example of output from soil tests from ten distinct zones of the bed, use graphic to understand Tables 1-2 above.
Comments:
I don't doubt it. The lack of rains this year is exacerbating the problem because we didn't get the magnitude of leaching we are accustomed to. It seems the big theme this year for berry growers is salt up and down the coast.
Appreciate you reading the blog by the way and sharing your thoughts.
Mark


Posted by Maxwell V. Norton on August 3, 2012 at 8:17 AM