Quick Tip on Interpreting Calcium Results on Soil Analyses
Another short summary from the Fertility Short Course held by Tim Hartz and Stuart Pettigrove at UC Davis this past fall.
Calcium (Ca) has an important role in plant nutrition, but we tend not to pay too much attention to it because rarely is it an issue in berry health on the Central Coast. As outlined further on in this post, it's because we have so much of it already in our soils here.
Crop uptake of calcium is in the range of 100- 300 lb per acre, which means having a soil test like the one below with something around 5100 ppm has a potential supply of 10,200 lbs of Ca per acre (5100 PPM x 2 = 10,200 lbs/A) in the top 6” of soil apparently is well excess of what is needed.
Then again, we know that all the Ca removed in this ammonium acetate (NH4OAc) extraction is not all available to the plant because it can be tied up on soil particles, organic matter, colloids or precipitated out as Ca compounds.
A saturated paste analysis like the one on the left of the soil test below (circled in green) is the way to address what is really available to the plant. Results are most commonly given in meq/l, meaning milliequivalents per liter solution.
To get a feel for the Ca availability that we see on our saturated paste soil results, bear in mind that hydroponic solutions for greenhouse or substrate production, which are formulated for optimum crop growth and production, are 4-8 meq Ca/liter, and comprise 30-50% of the total cation meq. Comparing this ideal formulation with a survey (see link below) of representative crop production soils in coastal and central California which found of average of approximately 6.6 meq/liter Ca (assuming a 5:1 ratio soil solution/saturated paste and equivalent to 660 ppm Ca) and represented 55% of the cation milliequivalents. This amount, roughly calculated out, arrives at 600 lb Ca in soil solution. To reiterate from the above, crop removal is around 100-300 lb per acre, so this gives us a lot of confidence that we really have ample Ca around here for our berry crops.
Thinking through this to its practical application in the field, compared to the large amounts of calcium already available in our soils, fertilizer additions of calcium don't really add that much at all. For example, the “calcium kick” from a fertilizer like CAN-17 (12.3 lb/gal, 8.8 % Ca, so a 3 gal/A application simply adds 3.25 lbs of Ca/A - a mere drop in the pool of 600 lbs already there for the plant) isn't much of a kick at all.
Here is the excellent link discussing calcium availability in coastal and central California soils referred to in this post :
http://www.cdfa.ca.gov/is/docs/04-0701Hartz%2007.pdf
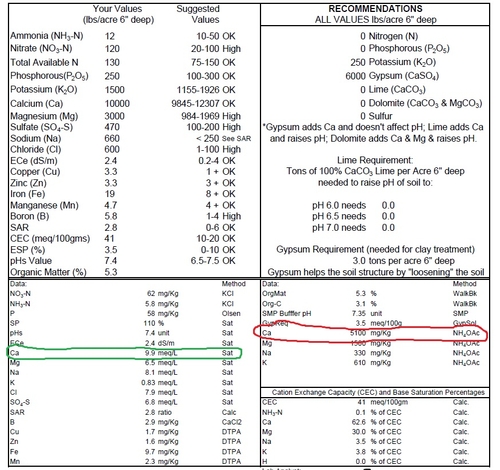
Soil sample from strawberry field taken last year. Potential calcium supply is 5100 ppm on the right and circled in red, and saturated paste, a measure of calcium availability, is 9.9 meq/l, is on the left circled in green.

